Fuel cells are innovative devices that convert chemical energy directly into electricity through an electrochemical reaction, producing clean and efficient power. Understanding how fuel cells work reveals their potential to revolutionize energy systems by using hydrogen as a primary fuel. There are several types of fuel cells, including proton exchange membrane and solid oxide, each suited for different applications. Modern hydrogen fuel cell technology powers vehicles, industries, and even remote power systems, offering a sustainable alternative to fossil fuels. When comparing fuel cell advantages and disadvantages, their high efficiency and zero-emission output stand out, though cost and durability remain challenges. Today, the applications of fuel cells continue to expand—from electric vehicles to backup power—marking a significant step toward a cleaner and more reliable global energy future.
Fuel Cells is a hot topic among scientists these days thanks to their wide range of applications. Their uses are so diverse that fuel cells have found a place even in the space program. In this blog, let me explain in detail the design, working, types and future scope of fuel cells.
Shall we begin?
Table of contents
How do fuel cells work ?
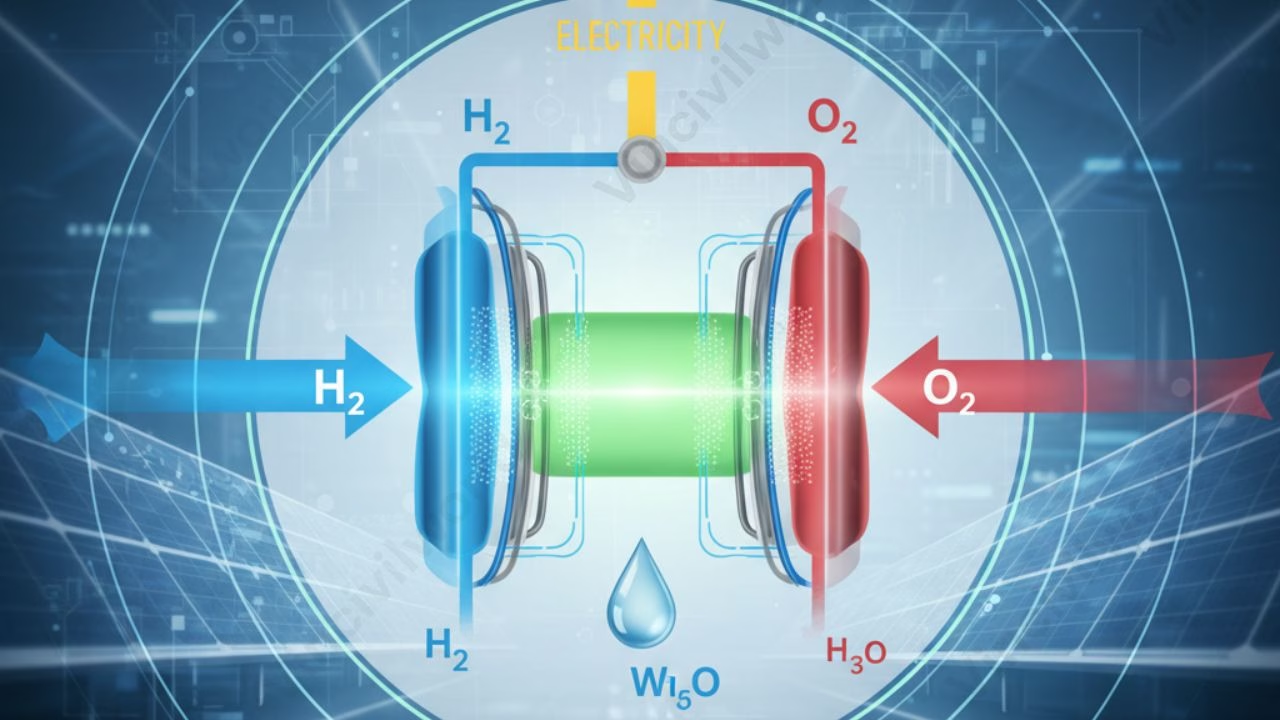
Fuel cells are electrochemical cells that use a pair of redox reactions to transform the chemical energy of a fuel (typically hydrogen) and an oxidizing agent (usually oxygen) into electricity. It finds various applications, including transportation, industrial/commercial/residential structures, and long-term grid energy storage in reversible systems.
Fuel cells are unique in that they may use a wide range of fuels and feedstocks and can power systems as large as a utility power plant and as small as laptop computers. Now, we are moving on to the design of fuel cells.
Also read: Solar Energy- Definition, Advantages, and Future
Design
A fuel cell comprises 3 adjacent segments namely the anode, the electrolyte, and the cathode. At the intersections of these segments, redox reactions take place. Fuel is burned, water or carbon dioxide is produced, and an electric current is produced, which can be utilized to power electrical devices, commonly referred to as the load.
A fuel cell’s design elements include:
- An electrolyte – It acts as a medium of transport between the electrodes. Most common electrolytes include potassium hydroxide, salt carbonates, and phosphoric acid, and it usually defines the type of fuel cell.
- A fuel – The fuel undergoes oxidation reaction and supplies the ions. Hydrogen is the most common fuel.
- Anode Catalyst – It breaks down the fuel into electrons and ions. We usually use fine platinum powder as the anode catalyst.
- Cathode catalyst – It reacts with the ions that reach the cathode and transforms them into harmless compounds, the most common of which is water.
- Gas diffusion layers that are resistant to oxidation.
Let me show you how fuel cells produce electricity from the fuel we supply.
Fuel Cells Working
In 1839, Sir William Robert Grove, a physicist invented the first fuel cell. The goal of a fuel cell is to generate an electric current that can do some work outside of the cell, such as powering an electric motor or lighting a city.
A catalyst at the anode promotes oxidation reactions in the fuel. As a result, hydrogen atoms are stripped of their electrons at the anode of a fuel cell. The hydrogen atoms have now become positively charged H+ ions.
At full rated load, a typical fuel cell produces a voltage of 0.6 to 0.7 V. If we require alternating current (AC), we must channel the DC output of the fuel cell via a conversion device called an inverter.
Reactions inside
When the ions and electrons reach the cathode, they rejoin, and the two react with a third molecule, usually oxygen, to produce water or carbon dioxide. The following are the basic reactions that take place inside a fuel cell:
Anode side: 2H2 => 4H+ + 4e–
Cathode side: O2+ 4H++ 4e–=> 2H2O
Net reaction: 2H2 + O2 => 2H2O
Different types of fuel cells
Depending on the electrolyte in use, there are different types of fuel cells. Here are some of them:
Alkali based
- Alkali fuel cells use compressed hydrogen and oxygen to function.
- Their electrolyte is usually a solution of potassium hydroxide (chemically, KOH) in water.
- The efficiency is around 70%, and the operating temperature is between 150 and 200 degrees Celsius (about 300 to 400 degrees F).
- The output of the cells ranges from 300 watts (W) to 5 kilowatts (kW).
- However, they require pure hydrogen fuel, and their platinum electrode catalysts are costly. They can also leak, just like any other liquid-filled container.
- In the Apollo spacecraft, alkali cells were employed to produce both electricity and drinking water.
Molten Carbonate based
- The electrolyte of molten carbonate (MCFC) consists of high-temperature salt carbonates (chemically, CO3).
- The efficiency ranges from 60% to 80%, and the working temperature is around 650°C (1,200 degrees F).
- The high temperature prevents the poisoning of cell by carbon monoxide, and waste heat can be recycled to generate more energy. However, the high temperature limits the materials and applications of MCFCs–they are likely too hot for domestic use.
- In addition, the processes consume carbonate ions from the electrolyte, necessitating the injection of carbon dioxide to compensate.
Also read: Tidal Energy – Definition, Advantages, and Future
Phosphoric Acid based
- The electrolyte of PAFCs is phosphoric acid, which is a non-conductive liquid acid that causes electrons to go from anode to cathode via an external electrical circuit.
- Since the anode’s hydrogen ion generation rate is low, we use platinum as a catalyst to boost the ionisation rate.
- The use of an acidic electrolyte is a major disadvantage of these cells. This accelerates the corrosion or oxidation of phosphoric acid-exposed components.
- The operating temperature is between 150 and 200 degrees Celsius, and the efficiency ranges from 40 to 80% (about 300 to 400 degrees F). Phosphoric acid cells now available have outputs of up to 200 kW.
Solid Oxide Fuel Cells
- Solid oxide (SOFC) use a hard, ceramic composition of metal oxides such as calcium or zirconium as an electrolyte.
- The efficiency is around 60%, and the output of the cells can reach 100 kW.
- The working temperature is around 1,000 degrees Celsius (about 1,800 degrees F).
- Further energy generation through waste heat recovery is possible. The high temperature, on the other hand, limits the applications of SOFC units, which are typically quite big.
Hydrogen fuel cell technology
Hydrogen fuel cell technology generates electricity through a chemical reaction between hydrogen and oxygen, with water and heat as the only byproducts. Inside the cell, hydrogen passes through the anode and splits into protons and electrons. The electrons flow through an external circuit, creating electricity, while the protons move through the electrolyte to combine with oxygen at the cathode. This process produces clean, efficient, and continuous power without combustion. Hydrogen fuel cells are used in electric vehicles, portable power systems, and large-scale energy storage. Their high efficiency and zero emissions make them a promising solution for reducing dependence on fossil fuels and advancing sustainable energy technologies worldwide.
Let’s have a look at the different applications of fuel cells.
Fuel Cells Applications
Fuel cell technology has a variety of applications. Currently, scientists are carrying out extensive research to develop a cost-effective powered automobile. The following are a few examples of the uses of this technology:
- Fuel cell electric vehicles, or FCEVs, use clean fuels and are thus more environmentally benign than vehicles powered by internal combustion engines.
- Many space voyages, like the Appolo space program, have relied on them for power.
- In many rural regions, fuel cells are a major backup source of electricity.
Also read: Wind Energy: Definition, Advantages, and Future
Fuel cell advantages and disadvantages
Fuel cell advantages and disadvantages highlight both the promise and challenges of this clean energy technology. Among the key advantages, fuel cells provide high energy efficiency, produce zero harmful emissions, and operate quietly, making them ideal for vehicles, power generation, and portable devices. They can continuously generate electricity as long as hydrogen is supplied, offering a reliable and eco-friendly alternative to fossil fuels. However, the disadvantages include high manufacturing costs, limited hydrogen availability, and complex storage and transport requirements. In addition, performance degradation over time remains a concern. Understanding fuel cell advantages and disadvantages helps guide innovation toward making this technology more affordable, accessible, and sustainable worldwide.
Fuel cells outperform traditional combustion-based technologies, which are now in operation in many power plants and automobiles. The advantages is that they emit fewer greenhouse gases and zero atmospheric pollutants that contribute to smog and health issues. When pure hydrogen is the fuel, the only by products are heat and water. Traditional combustion systems use significantly more energy than hydrogen-powered ones.
Fuel Cells Future
- Hydrogen abundance: Hydrogen is the most abundant element in the universe, making it a sustainable and renewable energy source for the future.
- Hydrogen ecosystem: A growing hydrogen ecosystem centered on fuel cell technology holds immense potential for clean energy innovation.
- Scalability: Unlike batteries, this technology can be scaled up for various transportation modes, including cars, buses, ships, and trains.
- Urban air mobility: Hydrogen will play a vital role in powering future air mobility solutions, such as drones and air taxis.
- Automotive transition: These could replace petroleum in vehicles, leading to zero-emission transportation.
- Industry leadership: Companies like Hyundai are pioneering hydrogen technology, expanding alongside battery, hybrid, and plug-in electric vehicle developments.
Key Takeaways
- Fuel cells convert chemical energy into electricity using hydrogen and oxygen in an electrochemical reaction, offering clean energy solutions.
- Different types of fuel cells exist, including alkali, molten carbonate, phosphoric acid, and solid oxide, each with unique designs and applications.
- Hydrogen fuel cell technology powers various applications, such as fuel cell electric vehicles and backup power sources in rural areas.
- Despite advantages like high efficiency and zero emissions, fuel cells face challenges such as high costs and limited hydrogen availability.
- The future of fuel cells looks promising with ongoing research and development aimed at enhancing technology for widespread use.
Conclusion
The future of fuel cells is filled with potential as industries seek cleaner and more efficient energy sources. With their non-polluting nature and wide range of applications, fuel cells stand out as a key player in sustainable development. However, challenges such as high production costs and safe, long-term hydrogen storage must be addressed to unlock their full potential. Continued advancements in hydrogen fuel cell technology and large-scale adoption could make fuel cells a cornerstone of global energy systems. Once cost and storage issues are resolved, these systems will not only revolutionize the energy sector but also help achieve a cleaner, greener, and more reliable energy future for generations to come.
In case of any queries, please feel free to ask in the comments section. Happy Learning!