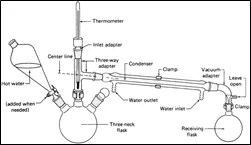
Steam distillation is a separation process in which we separate a mixture of immiscible components by introducing steam and subsequently condensing the vapours. In this blog, I will walk you through steam distillation and its principles. First, let us understand the instances in which we opt for Steam distillation over other separation processes.
Table of contents
What is Steam Distillation?
In the typical distillation process, we usually have a mixture of components that are miscible with one another. The vapour pressure that the combination exerts on heating depends on the components that make up the mixture.
For boiling to start, the vapour pressure of the mixture must equal the atmospheric pressure. It can also equal the pressure to which it is subjected to. We must heat the system of the liquid mixture to a higher temperature. At this temperature, the system can create enough vapor to equalize the operating pressure or the atmospheric pressure.
The temperature that must be attained depends on the operating pressure. If it is less than one atmospheric pressure, the temperature to be attained is relatively lower. If it is greater than one atmospheric pressure, the temperature to be attained is relatively higher.
In some circumstances, it might not be possible to perform this. Some of those instances are as follows:
- When separating materials with very high boiling points, we have to supply more heat. This raises the temperature of the mixture. As a result, the procedure uses more energy and is more expensive.
- If the mixture contains any thermally unstable components, high temperatures could cause decomposition. This decomposition affects their qualities.
- The process becomes energy-intensive in certain conditions. This occurs when one component in a binary combination boils at a high temperature. Meanwhile, the other component is non-volatile in nature.
- We can easily handle these situations using the method of steam distillation.
Also read : Cable stayed bridges
Steam Distillation Principle
In the previous blog, we saw Raoult’s law. It states that the partial pressure of each component in a miscible ideal mixture is equal to the product of its vapour pressure and mole fraction.
Pa = Xa * Pv
Hence it is clear that the liquid components can’t exert their actual vapour pressure but a corrected vapor pressure (or what we call the partial pressure) which is always less than its pure component vapour pressure ( since mole fraction is always less than 1 )
But, in the case of non-miscible liquid mixtures, the components can exert their entire vapour pressure. This becomes its partial pressure. That is, the total pressure becomes equal to the sum of the individual vapour pressures for immiscible liquid mixtures. Their combined vapour pressures can easily reach the external pressure. This happens before the vapour pressure of either of the individual components crosses it. Hence the boiling point of the mixture would be lesser than the boiling point of either of the components.
Now, let us assume that water is one of the components in the immiscible mixture. We can bring that mixture to a boil at under 100 0C in one atmosphere. This is true if we keep the pressure constant at 1 ATM. The boiling point of water at 1 ATM is 100 0C. In other words, we can lower the operating pressure needed to boil the mixture by introducing steam.
We use steam to create pressure in steam distillation. This pressure helps balance the operating pressure. We must be careful to only employ components that are immiscible with water while using steam.
Steam Distillation Process
Consider a binary mixture where component A is a high-boiling component and component B is a non-volatile component. Let’s say A is insoluble in water. We feed the mixture into the column. Using a steam coil, we raise the feed mixture’s temperature. A sparger forces the steam through another steam line. Steam enters the column through the feed mixture and adds to the vapour pressure. When it reaches the working pressure, it causes the creation of vapours of A at a significantly lower temperature. The non-volatile component is eliminated as residue but remains in the feed. Steam and Component A is routed via a condenser where they are easily separated after condensation.
Steam Distillation Advantages
We frequently use steam distillation since it has various advantages over other extraction methods. They are as follows:
- the process produces organic compounds devoid of solvents;
- Additional separation procedures are not necessary;
- It has a huge processing capacity on an industrial scale;
- Inexpensive equipment
Shall we wrap up?
Conclusion
Steam distillation is an efficient and versatile separation process. It is used to extract immiscible components. This is especially beneficial for those with high boiling points or thermal instability. By introducing steam, it reduces the boiling point of the mixture, conserving energy and preventing decomposition of sensitive materials. This method is widely applied in industries for its cost-effectiveness, simplicity, and ability to produce pure compounds without solvents. Understanding its principles leads to better application in industrial settings. It also enhances use in laboratory settings. This knowledge makes it an essential process in chemical engineering and related fields.
Key Takeaways
- Operates efficiently at reduced temperatures with minimal environmental impact.
- Steam distillation separates immiscible components using steam and condensation.
- It reduces boiling points, conserving energy and avoiding thermal decomposition.
- Effective for extracting high-boiling or thermally unstable compounds.
- Requires immiscible components for successful operation.
- Simplifies processes by eliminating the need for solvents and additional separation steps.
- Used in various industries due to cost-efficient equipment and high processing capacity.